By Samuli Stromberg
|

|
|
Photo by UPM Raflatc |
STOPPING COUNTERFEIT DRUGS
The U.S. Congress passed drug pedigree law eighteen years ago in order to guarantee the authenticity of drugs. However, some of the rules for implementing the law were never finalized and loopholes left the door open for counterfeit medicines to enter the mainstream medicine supply and the shelves of trusted local pharmacies.
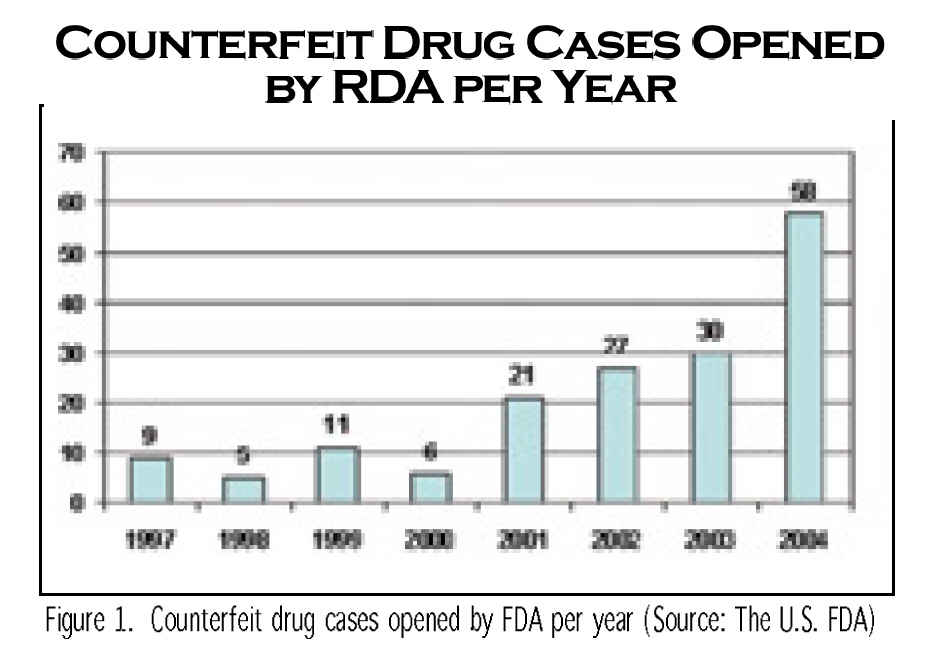
Drug counterfeiting is a US$32-billion-a-year business, and as much as ten percent of drugs worldwide are counterfeit. According to World Health Organization estimates, the business has grown 30 to 50 percent annually. Counterfeit drugs look the same as the real thing, but do not contain the essential active ingredient or might have some contaminants.
Increased control of the logistics chain aims at preventing counterfeit drugs from ending up in consumers¡¯ hands. The new Pedigree regulation will ensure the tracking and authentication of pharmaceuticals. In practice, this means drug distributors are required to document the chain of custody as drugs move through the distribution channel. Each individual package or bottle needs ID information so that it can be identified on its way from factory to the consumer. Information includes confirmation that the product is genuine, has not passed its expiry date and is not subject to a recall.
The FDA has recently reaffirmed its recommendation that Radio Frequency Identification (RFID) technology be used widely throughout the pharmaceutical industry by 2007. The FDA is encouraging the pharmaceutical industry to adopt RFID as a tracking technology to create electronic pedigrees, or ePedigrees. Technically, the FDA is mandating the use of electronic track and trace technology, of which RFID is the most widespread application.
US TO ADOPT RFID FIRST
¡±The U.S.A. represents sixty percent of the pharmaceutical industry worldwide. Decisions made in the U.S.A. will therefore eventually affect European legislation and practices. Counterfeiting is a big problem even in Europe,¡± says Samuli Stromberg, VP Marketing, RFID at UPM Raflatac.
¡±The U.S.A. will most likely be the first to adopt RFID in the pharmaceutical industry. The strongest drivers for implementing RFID are in the U.S.A. because of the FDA¡¯s decision to implement Pedigree. They also have the most homogenous legislation regulating the pharmaceutical industry.¡±
¡±The pharmaceutical industry is a vast market where changes do not happen overnight; one year is a short time. However, it is likely that all major companies will at least test RFID technology,¡± explains Stromberg. He also notes that the pharmaceutical industry is still debating two differing RFID technologies -- newer long-range Ultra High Frequency (UHF) and High Frequency (HF), which has been around for years.
TRACKING ALL THE WAY
Utilizing RFID technology offers a number of advantages for pharmaceutical companies with complex international delivery chains. With RFID, drug identification can be achieved reliably and rapidly at each point of the supply chain, all the way from the factory to the consumer. There is no need for huge investments or additional manual labour.
An electronic pedigree with complete tracking history staves off counterfeit products and grey distribution channels. As authentication makes counterfeiting difficult, it also serves to protect brand value. In addition, tracking can improve the process efficiency of each supply chain partner by making receiving and delivering goods easy and fast. RFID is also a tool for a quick and efficient product recall management process. Companies testing RFID have praised the system for its merits in supply chain collaboration and open data sharing, where authorized parties are allowed to monitor the documented pedigree information.
RFID FOR THE PHARMACEUTICAL INDUSTRY
Many of the global top ten drug manufacturers either have deployed RFID projects, or are in the process of doing so. Some U.S. drug companies have already initiated their own rollouts of RFID to track and trace their products, most notably Pfizer, maker of Viagra. Drug manufacturer GlaxoSmithKline Inc. has also designed its RFID supply chain project. These high-profile pharma RFID pilots are not only for ePedigree but also for business improvements, ROI-driven adoption.
UPM Raflatac is a partner in many of the RFID pilots ongoing in the pharmaceutical industry. In a trial involving 44 UK pharmacies, for example, Aegates RFID and bar code tagging system helped reduce dispensing errors and intercept counterfeit drugs. Aegate tagged products from six drug manufacturers including Merck, Novartis and Schering Health Care.
UPM Raflatac has also supplied the tags for the pilot of Finnish drug manufacturer Orion Pharma. This pilot is unique in Europe as it involves all the different parties in the supply chain and even the package manufacturer. The RFID technology proved itself during trials. According to Orion, tagging, tracking and authentication of individual items was successful, and the companies gained an insight into how to use RFID to track raw materials and packaging in their own manufacturing processes. Orion says it will start item-level RFID tagging at some point in the future.
It is easy to deploy item-level tagging with HF RFID tags, as the short read range is appropriate for use in the pharmacy, especially at the point of sale. UHF RFID tags are outstandingt for case or pallet level tracking as the read ranges are longer.
When deploying RFID, you need to ensure the materials, packages, packaging hierarchy and positioning of tags are suitable for HF or UHF tags. Furthermore, the whole data administration and systems used have to be adjusted to accommodate RFID use, including rules for access and modification rights.
WHAT ARE RFID TAGS
Radio Frequency Identification is a method of identifying unique items using radio waves. An RFID tag consists of a microchip and an antenna that is packaged in a way that allows it to be applied to an object. The tag enables product authentication and follow-up of product flow and stock levels. An encoded tag, which includes product ID information, is attached to each product or package. When a tagged item is brought in proximity of a reader, the reader recognizes the tag and starts sending radio signals that activate the tag to send the ID data of this specific product to the reader. This information is then passed on to an information system to be processed.
RFID TAGS VS BAR CODES
RFID tags can be embedded in the labels or inserted inside drug bottle caps and packages and scanned like bar codes. However, the tags store more information than bar codes and can be scanned from further away. In addition, and unlike bar codes, several of them can be scanned simultaneously. In retail, major companies such as Wal-Mart and their suppliers such as Procter & Gamble have agreed on standards for tags and to push for increased adaptation.
Samuli Stromberg is Vice President of RFID Marketing at UPM Raflatac (www.upmraflatac.com).
For more information, please send your e-mails to swm@infothe.com.
¨Ï2007 www.SecurityWorldMag.com. All rights reserved.
|